How Fast is Your Skin Aging? Your Skin Microbiome Can Tell You.
by Parallel Health Team
Scientists have coined a term to describe a fundamental driver of aging: "inflammaging"—the combination of inflammation and aging. Research shows that older adults develop a pro-inflammatory state, driven by genetic predisposition, compromised gut permeability, microbial imbalances, oxidative stress, and cellular senescence.
Your skin microbiome both contributes to and reflects this inflammatory cascade.
The Microbial Shift That Accelerates Aging
Microbial shifts occur with aging and may accelerate age-related skin changes. As we age, disturbances in the gut microbiome intensify inflammaging by promoting senescent cell accumulation and weakening immune response. On the skin itself, bacterial diversity increases with age at all skin sites (note that bacterial diversity can be bad or good; it all depends on the composition of that diversity), while specific beneficial genera like Lactobacillus and Cutibacterium show significant decreases in abundance.
This isn't merely correlation. Gut dysbiosis with age results in leakage of proinflammatory microbial products through compromised intestinal permeability, which then enter the bloodstream and create systemic effects. Bacterial antigens, especially lipopolysaccharide, increase circulating pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6, contributing to chronic low-grade systemic inflammation.
This process results in a self-perpetuating cycle where microbial dysbiosis drives inflammation, inflammation accelerates cellular aging, and aging further disrupts microbial balance.
The Barrier Breakdown
In aged skin, repair mechanisms become impaired, leading to prolonged barrier disruption that creates persistent elevation of proinflammatory cytokines and chronic inflammation. Research demonstrates that elevated cytokine levels in both serum and skin of aged mice can be normalized through daily barrier improvement via epidermal rehydration, suggesting that skin-derived signals play a significant role in systemic inflammatory markers.
This reveals a critical insight: the skin barrier and its microbial ecosystem aren't merely cosmetic concerns. They're fundamental to systemic inflammation and the aging process itself. But the good news is that you can improve in both of these areas with the right personalized approach.
Precision Intervention: Engineering the Microbiome for Longevity
At Parallel Health, we've developed Microbiome Dermatology™ (offered via The MD-03 Protocol™)—a precision approach to skin longevity that begins with understanding your unique microbial landscape. Using quantitative whole genome sequencing of your skin microbiome, we identify specific dysbioses that may be driving chronic inflammation.
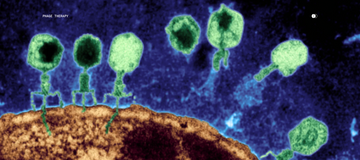
The intervention is equally precise: bacteriophage therapy. Phages exhibit high specificity for their targeted bacteria, making phage-replacement therapy a promising strategy for controlling pathogenic bacteria. Unlike antibiotics that indiscriminately eliminate microbial populations, bacteriophages specifically target bacteria, making them an effective option for reducing pathogenic bacterial populations without disrupting beneficial microbes.
Recent analysis revealed significantly increased prevalence of C. acnes phages in healthy individuals compared to those with acne, linking phage abundance to healthy skin. This suggests that phage therapy doesn't just treat symptoms—it restores the natural regulatory mechanisms that maintain microbial equilibrium.
This is skin microbiome engineering for skin longevity: identifying the specific microbial imbalances driving your chronic inflammation, then using targeted phage therapy to restore balance without the collateral damage of conventional treatments.
Because looking younger and maintaining cellular health aren't separate objectives. When you address inflammaging at its microbial source, you're not choosing between aesthetics and biology—you're recognizing they're fundamentally interconnected.
Frequently Asked Questions
What is inflammaging and why does it matter for skin?
Inflammaging is the chronic, low-grade inflammation that develops with age. This pro-inflammatory state contributes to age-related conditions and involves factors including microbial composition changes, oxidative stress, and cellular senescence. For skin specifically, inflammaging drives tissue degeneration as pathogenic species release toxins and inflammatory signals, creating a sustained immune response. This accelerates visible aging and compromises skin barrier function. Parallel's Skin Discovery Test™ can uncover your skin age, and your Quantitative Microbial Analysis™, which comes with every test, reveals the reasoning behind that score.
How does the skin microbiome change with age?
We know that there are age-dependent changes in microbial communities. Some of these observed changes potentially affect skin immune barrier integrity, biosynthesis of age-related substances, and contribute to chronic inflammatory stimulation. That is, some bacteria accelerate skin aging; some decelerate skin aging.
What connects gut health and skin aging?
The gut-skin axis represents bidirectional communication between the gut microbiome and skin. Gut dysbiosis leads to increased intestinal permeability, allowing proinflammatory microbial products to enter the bloodstream and cause systemic effects. These inflammatory signals contribute to skin aging, barrier dysfunction, and conditions like acne, eczema, and rosacea.
How is phage therapy different from antibiotics?
Bacteriophages are nano-microbes that specifically target bacteria, allowing them to reduce pathogenic bacterial populations without disrupting beneficial microbes. Antibiotics work indiscriminately, eliminating both beneficial and harmful bacteria, which can worsen dysbiosis. Phages offer host specificity and simplicity of isolation and production, making them an attractive alternative as antibiotic resistance increases. Parallel's Skin Discovery Test™ also provides an antibiotic resistance score, which measures how many of your bacteria are resistant already to known antibiotics.
How does Parallel Health's approach work?
Parallel Health uses quantitative whole genome sequencing to analyze your skin microbiome composition, identifying specific bacterial imbalances that may be contributing to inflammation and accelerated aging. Based on this analysis, we develop personalized phage therapy formulations that selectively target problematic bacterial strains while preserving beneficial microbial communities. This precision approach addresses the underlying cause of chronic inflammation rather than merely treating symptoms.
What is the evidence that phage therapy works for skin?
Recent cosmetic clinical trials, including those at Parallel Health, demonstrated that topical bacteriophages targeting C. acnes significantly reduced facial C. acnes compared to vehicle control and was safe and well-tolerated in subjects with mild-to-moderate acne. Studies have shown promising results for phage therapy in treating acne, psoriasis, and atopic dermatitis, though additional research is needed to validate efficacy and determine optimal administration mechanisms.
Can I use phage therapy alongside other skincare treatments?
Yes. Phage therapy targets specific bacterial populations and can be used alongside topical treatments like peptides, retinoids, or other active ingredients. In fact, combining phage therapy with barrier-supporting ingredients may provide synergistic benefits—addressing both microbial dysbiosis and barrier function simultaneously.
How long does it take to see results from microbiome-based interventions?
The timeline varies depending on the severity of dysbiosis and individual factors. Clinical evidence supports that both oral and topical administration of probiotics, postbiotics, and phage therapy contribute to wrinkle reduction and improved skin texture in older adults. Most users see improvements in inflammation and barrier function within 4-12 weeks, with continued benefits as microbial balance is restored.
Is skin microbiome testing worth it?
Individual microbiomes vary significantly, and in designing phage therapy, the composition of individual microbiome structure should be taken into consideration. Generic "one-size-fits-all" approaches miss the precision that makes microbiome interventions effective. Testing identifies your specific dysbioses, allowing for targeted treatment rather than empirical approaches.
References
[1] Woo YR, Lee SH, Cho SH, Lee JD, Kim HS. Interaction between the microbiota and the skin barrier in aging skin: a comprehensive review. Front Physiol. 2024;15:1322205.
[2] Shen CY, Hsu CY, Liao YM, Chen LW, Lin TC, Lu CY, Ko WC, Chen YH. How Microbiomes Affect Skin Aging: The Updated Evidence and Current Perspectives. Clin Cosmet Investig Dermatol. 2022;15:1455-1476.
[3] Somboonna N, Wilantho A, Srisuttiyakorn C, et al. New Insights Into the Skin Microbial Communities and Skin Aging. Front Microbiol. 2020;11:565549.
[4] Swaney MH, Kalan LR. Aging-Associated Changes in the Adult Human Skin Microbiome and the Host Factors that Affect Skin Microbiome Composition. J Invest Dermatol. 2022;142(5):1444-1450.
[5] Kim HJ, Jeong M, Choi SK, Kim H, Park M, Jung E. Microbiome-Based Interventions for Skin Aging and Barrier Function: A Comprehensive Review. Ann Dermatol. 2025;37(1):1-13.
[6] Thakur R, Ghosh A, Nag M, Patel D. Microbiome–Aging–Wrinkles Axis of Skin: Molecular Insights and Microbial Interventions. Biomedicines. 2025;13(1):122.
[7] Castillo DE, Nanda S, Keri JE. Propionibacterium (Cutibacterium) acnes Bacteriophage Therapy in Acne: Current Evidence and Future Perspectives. Dermatol Ther (Heidelb). 2019;9(1):19-31.
[8] O'Neill AM, Nakatsuji T, Hayachi A, Williams MR, Mills RH, Gonzalez DJ, Gallo RL. Identification of a Human Skin Commensal Bacterium that Selectively Kills Cutibacterium acnes. J Invest Dermatol. 2020;140(8):1619-1628.
[9] Jończyk-Matysiak E, Weber-Dąbrowska B, Górski A. Prospects of Phage Application in the Treatment of Acne Caused by Propionibacterium acnes. Front Microbiol. 2017;8:164.
[10] Verbanic S, Shen Y, Lee J, Deacon JM, Chen IA. Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes. 2020;6(1):21.
[11] Graham MT, Collie D, Seale AC, Barr DA. Bacteriophages and the Microbiome in Dermatology: The Role of the Phageome and a Potential Therapeutic Strategy. Int J Mol Sci. 2023;24(3):2695.
[12] Shamriz S, Alebouyeh M, Karami P, Mosaffa N, Taghizadeh S, Vaziri F. The Role of the Skin Microbiome in Acne: Challenges and Future Therapeutic Opportunities. Int J Mol Sci. 2024;25(21):11422.
[13] Palacios-García L, Leech J, Townsend E, Paddock S, Mishra V, Ferry D, Ramage G, Blanco-Míguez A, Russell RK, Segal JP, Quince C, Ijaz UZ, Hansen R. Development of a topical bacteriophage gel targeting Cutibacterium acnes for acne prone skin and results of a phase 1 cosmetic randomized clinical trial. Skin Health Dis. 2022;2(3):e125.