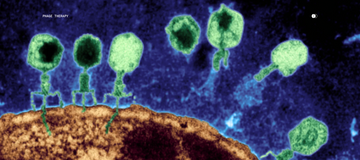
The CDC estimates that 47 million antibiotic prescriptions each year—nearly one in three—are unnecessary. And dermatologists are the leading prescribers.
But emerging research suggests these prescriptions may carry metabolic consequences that extend far beyond the skin.
Recent longitudinal studies tracking over 430,000 children have identified a modest but consistent association between early antibiotic exposure and increased weight trajectories. This association reveals something fundamental about how we've been disrupting one of our body's most sophisticated regulatory systems.
The Microbiome-Metabolism Connection
Antibiotics don't simply kill bacteria; they reshape entire ecosystems. When broad-spectrum antibiotics course through your body, they indiscriminately eliminate both pathogenic and commensal bacteria, fundamentally altering the metabolic capacity of the microbiome.
This disruption manifests through several interconnected pathways. Beneficial gut bacteria can produce critical metabolites, also called short-chain fatty acids (SCFAs), which regulate energy expenditure, appetite signaling, and insulin sensitivity. Antibiotics can kill these beneficial bacteria and can also induce further dysbiosis post-treatment, reducing SCFA production and effectively recalibrating the body's metabolic set point. Simultaneously, changes in bile acid metabolism alter fat absorption and hormonal signaling cascades that regulate weight.
The intestinal barrier itself can also become compromised. Because beneficial bacteria decline, inflammatory molecules can begin to enter systemic circulation. This low-grade inflammation—"metainflammation"—creates conditions conducive to metabolic dysfunction and weight gain.
Additionally, certain bacterial populations are more efficient at extracting calories from food. Antibiotics can shift the gut's taxonomic composition toward these "obesogenic" profiles, effectively increasing energy harvest from the same dietary intake. For example, a healthy microbiome might extract 250 calories from a 300-calorie cookie, leaving the rest undigested. But antibiotic-disrupted bacteria can be more efficient, extracting 280 calories from that same cookie. This difference compounds with every meal—you're gaining weight not because you're eating more, but because your microbiome is harvesting more energy from the same diet.
The One-Third Problem
Paradoxically, approximately one-third of all antibiotic prescriptions are medically unnecessary, according to the CDC. In dermatology, where antibiotics have been prescribed for decades as first-line acne treatment, we've normalized a practice that may carry unintended metabolic consequences—particularly when repeated courses become routine.
This isn't an argument against antibiotics when medically indicated. Rather, it's a recognition that the threshold for "indicated" deserves recalibration in light of emerging evidence about microbiome disruption, antibiotic resistance, and metabolic health.
Precision as an Alternative
Quantitative metagenomic sequencing, like that pioneered by Parallel Health, can now identify specific pathogenic strains driving skin conditions, enabling targeted interventions that preserve beneficial microbiota. Then, bacteriophage therapy—nano-microbes that infect only specific bacterial species can eliminate pathogens without collateral damage to the broader ecosystem.
Parallel also offers custom-compounded formulations, informed by individual microbiome profiles and clinical history, which offer a different mechanism of action for those who need extra support. These approaches require more upfront investment of time and energy due to the process of testing, analysis, and formulation development. The MD-03 Protocol™ isn't as immediate as a standard antibiotic prescription.
But convenience and optimal outcome don't always align. The microbiome represents decades of evolutionary dialogue between host and microbial communities. Disrupting it has consequences we're only beginning to quantify. For patients seeking sustainable solutions—particularly those with recurring conditions requiring repeated antibiotic courses—precision microbiome analysis may represent not just an alternative, but a more sophisticated understanding of the systems we're attempting to treat.
The question isn't whether antibiotics work. It's whether we can do better.
Frequently Asked Questions About the MD-03 Protocol™
What is the MD-03 Protocol™?
The MD-03 Protocol™ is Parallel Health's complete, personalized plan to promote skin longevity and support skin issues. It combines quantitative whole genome sequencing of your skin microbiome with custom-formulated bacteriophage therapy and compounded prescriptions tailored to your unique microbial profile. The protocol can support you wherever you are in your journey, whether you have a complex, chronic issue or already healthy skin.
How does skin microbiome testing work?
We use quantitative whole-genome sequencing—not just 16S rRNA sequencing—to identify the specific bacterial strains on your skin at species and strain level. This precision enables us to determine exactly which pathogens are driving your condition and which beneficial bacteria need to be preserved.
What is bacteriophage therapy?
Bacteriophages (or "phages") are nano-microbes that infect only specific bacterial species. Unlike broad-spectrum antibiotics that eliminate both harmful and beneficial bacteria, phage therapy targets only the pathogenic strains identified in your microbiome test, preserving your skin's healthy bacterial ecosystem.
How is this different from getting an antibiotic prescription?
Traditional antibiotics are one-size-fits-all and indiscriminately kill many different bacteria, pathogenic and beneficial. The MD-03 Protocol™ provides testing, alongside your personalized phage serum based on your specific microbiome composition. This precision approach avoids the metabolic disruption and resistance concerns associated with broad-spectrum antibiotics.
How long does the process take?
After collecting your skin sample, microbiome analysis typically takes 3-4 weeks. Your custom phage serum formulation is then compounded based on your results. While this is longer than filling a standard antibiotic prescription, the personalized approach aims for more sustainable, long-term outcomes.
Is this approach suitable for chronic or recurring conditions?
Yes—in fact, patients with recurring conditions who have completed multiple antibiotic courses may particularly benefit from the MD-03 Protocol™. Repeated antibiotic use can create increasingly dysbiotic microbiomes and contribute to antibiotic resistance, making precision microbiome-based treatment a more strategic long-term solution.
Does insurance cover the MD-03 Protocol™?
The MD-03 Protocol™ is HSA/FSA eligible, but not currently covered by insurance providers. However, many patients find that the long-term value of avoiding repeated antibiotic courses and addressing root causes justifies the investment.
Is the MD-03 Protocol™ a "subscription," and can I buy a custom phage serum a la carte?
The MD-03 Protocol™ is a complete plan that works like a subscription. There is a minimum commitment of three months to complete the introductory period of testing, analysis, "resetting your skin," and receiving your first custom phage. Custom phage serums cannot be purchased a la carte because your specific skin microbiome composition must be understood in order to formulate the right phage therapy for you—precision treatment requires precision diagnostics. However, patients can purchase the Skin Discovery Test a la carte if interested in understanding skin microbiome before committing to the full protocol.
References
Bailey, L.C., et al. (2022). Antibiotics prior to age 2 years have limited association with preschool growth trajectory. International Journal of Obesity, 46(4), 843-850. https://doi.org/10.1038/s41366-021-01023-w
Chang, J., et al. (2023). Acne accounts for an almost 2.5-fold higher proportion of dermatology visits among adult females compared to adult males in the United States. PLoS ONE, 18(9), e0290763. https://doi.org/10.1371/journal.pone.0290763
Saheb Kashaf, S., et al. (2023). Staphylococcal diversity in atopic dermatitis from an individual to a global scale. Cell Host & Microbe, 31(4), 578-592. https://doi.org/10.1016/j.chom.2023.03.010
Yang, S., et al. (2024). Prevention and treatment of antibiotics-associated adverse effects through the use of probiotics: A review. Journal of Advanced Research, 71, 209-226. https://doi.org/10.1016/j.jare.2024.06.006
CDC. (2016). 1 in 3 antibiotic prescriptions unnecessary. Retrieved from https://www.cdc.gov/media/releases/2016/p0503-unnecessary-prescriptions.html